PG-Diploma
In
Pharmacovigilance (PGDPV)
12 Months Program
Learning Mode:
Both Residential and Distance learning modes are available, Each offering equal value, flexibility, and recognition.
Residential (Classroom)

Distance (Online)
Course Description:
The Post Graduate Diploma in Pharmacovigilance (PGDPV) is a rigorous one-year academic program designed to equip students with comprehensive knowledge and applied skills in the field of drug safety and pharmacovigilance. Structured over four semesters of three months each, this program offers an in-depth curriculum that bridges the gap between theoretical understanding and practical execution in real-world safety monitoring settings within the pharmaceutical, biotech, and healthcare sectors.
The course is divided into 16 detailed modules. It begins with core concepts such as the history, scope, and principles of pharmacovigilance, followed by adverse drug reaction (ADR) management, signal detection, risk assessment, and case processing. As students advance, they are introduced to global regulatory frameworks, medical coding (MedDRA, WHO-ART), aggregate reporting (PADER, PSUR, DSUR), E2B standards, and safety database management. The final semesters focus on auditing, compliance, Good Pharmacovigilance Practices (GVP), and the integration of pharmacovigilance into clinical trials and post-marketing surveillance.
Through interactive sessions, real-world case studies, and exposure to industry tools and software, the program prepares students to confidently handle pharmacovigilance operations and regulatory submissions. PGDPV graduates emerge as industry-ready professionals who are capable of contributing to global drug safety and public health.
At the end of each semester, candidates must appear for an internal assessment (semester examination). A minimum score of 50% is required to successfully pass and progress to the next semester. This evaluation ensures continuous learning, concept clarity, and academic discipline throughout the program.
Course Curriculum:
SEMESTER I
- Module 1: Introduction to Pharmacovigilance
- Module 2: Drug Development &Safety Monitoring
-
Module 3: Adverse Drug Reactions (ADRs) &
Reporting Systems - Module 4: Case Processing Fundamentals
SEMESTER II
- Module 5: Global Pharmacovigilance Regulations & Guidelines (ICH, GVP, USFDA, EMA, CDSCO)
- Module 6: MedDRA & WHO-ART Medical Coding
- Module 7: Safety Databases & E2B Submission Standards
- Module 8: Signal Detection and Risk Management
SEMESTER III
- Module 9: Aggregate Safety Reporting (PADER, PSUR, DSUR)
- Module 10: Pharmacovigilance in Clinical Trials vs. Post-Marketing
- Module 11: Individual Case Safety Report (ICSR) Narratives & Quality Review
- Module 12: Introduction to Literature Screening & Triage
SEMESTER IV
- Module 13: Good Pharmacovigilance Practices (GVP) & Audit Readiness
- Module 14: Pharmacovigilance Quality Management Systems
- Module 15: Pharmacovigilance & Artificial Intelligence Integration
- Module 16: Case Studies, Project Work & Final Review
Eligibility Requirements
Educational Qualifications
- Bachelor’s and Master’s degree in Life Sciences or related fields | B.Pharm | M.Pharm | BDS | B.A.M.S|
- B.Sc Nursing | B.Sc Biotech | M.Sc Biotech | Microbiology | Zoology| Medicine | Biochemistry | M.B.B.S
Candidates who have passed with a minimum of 50% Graduate or Postgraduate + Internal Assessment + P. Interview

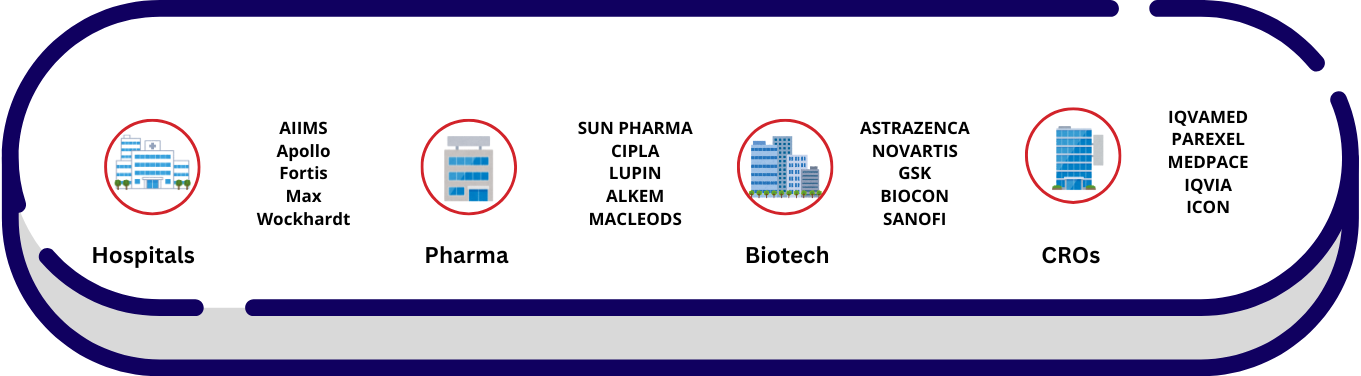
Industries Involved
Pharmaceutical:
- e.g., Drug development
Biotechnology
- e.g., Medical product innovation
Medical Device Companies
- e.g., Development and testing of devices like implants, diagnostic tools
CROs
- Contract Research Organizations for outsourcing research

Pharmacovigilance Market Size & Trends
Global Market Overview:
- Market Size (2023): USD 7.32 Billion
- Projected Growth (2024-2030): CAGR of 6.8%
Key Drivers for Market Growth:
Rising Incidence of Adverse Drug Reactions (ADRs):
- Due to drug abuse and the prevalence of diseases requiring combination drugs
- Increase in Novel Drug Production
Stringent Government Regulations:
- U.S. FDA & EU EMA regulatory guidelines for all phases of clinical trials
Advancements in ADR Databases & Information Systems:
- Accurate reporting and further use in clinical studies

Rise in Chronic Diseases & Drug Consumption
Increase in Chronic Diseases:
- Cancers, diabetes, cardiovascular, and respiratory disorders
- Higher drug consumption globally
WHO Report:
- Chronic disease treatments make up a large portion of drug consumption in non-hospital settings
Increasing Drug Development Activities:
- Personalized medicines, biosimilars, orphan drugs, companion diagnostics
- Adaptive trial designs projected to boost demand for pharmacovigilance services
Impact of ADRs & Drug Toxicity on Market Growth
ADR Incidence:
- NCBI: ~5% of hospitalizations in Europe due to ADRs
Serious ADRs in the U.S.:
- Over 100,000 deaths annually
- A major health concern since the past decade


Adverse Drug Events (ADEs) in Hospitals
Hospital Settings:
Inpatient:
- ADEs account for 1 in 3 hospital adverse events
- Affects ~2 million hospital stays annually
- Prolongs hospital stays by 1.7 to 4.6 days
Outpatient:
- Over 3.5 million physician office visits
- ~1 million emergency department visits
- ~125,000 hospital admissions
Market Concentration & Characteristics
Current Market Growth:
- The pharmacovigilance market is growing at an accelerating pace.
Growth Drivers:
- Rising Drug Consumption & Development Rates
- Higher Incidence of Adverse Drug Reactions (ADR) & Drug Toxicity
- Increasing Outsourcing Trend in Pharmacovigilance Services
Outsourced Services Include:
- Medical Writing
- Clinical Trial Data Collection
- Medical Reporting
- Other Pharmacovigilance-Related Services
Industry Trend:
- Manufacturers shifting from fully integrated pharmaceutical companies to collaborative partnerships with service providers to reduce costs and operational expenses.


India Pharmacovigilance Market Trends
Fastest Growing Market in Asia Pacific:
- Rising ADR incidence and growing healthcare professional awareness
Government Action:
- Central Drugs Standard Control Organization (CDSCO)
- Issued draft guidance on pharmacovigilance requirements for human vaccines
- Emphasizes monitoring, risk management, audits, inspections, and safety assessments
Impact on Market Growth:
- Continuous vigilance for vaccine safety boosts market developmen
Workspaces of PV Personnel
Where PV Professionals Work?
Types of Roles in PV Domain
Graduates of the PGDPV program can pursue roles such as:
At Hospital_the Investigative Site
The location(s) at or from where trial-related activities are conducted under the investigator’s/institution’s supervision.
- Principal Investigator (PI)
- Sub-Investigator (Sub-I)
- Clinical Research Coordinator (CRC)
- Clinical Research Nurses (CRN)
- Clinical Research Pharmacists (CRP)

Types of Roles in PV Domain
Drug Safety
Sponsor |CRO| Safety
- Director of Pharmacovigilance/Safety
- Drug Safety Manager
- Pharmacovigilance (PV) Officer
- Drug Safety Associate
- Senior Drug Safety Associate
- Pharmacovigilance Specialist
- Drug Safety Physician
- Safety Data Analyst
- Pharmacovigilance Scientist
- Medical Safety Officer
- Signal Detection Specialist
- Case Processing Specialist
- Adverse Event Reporting Coordinator
- Risk Management Specialist
- Safety Compliance Officer
- Safety Surveillance Manager
- Aggregate Reporting Specialist

Why Choose PGDPV at IILS?
Industry-focused curriculum aligned with regulatory standards.
Expert faculty with extensive clinical research experience.
Placement assistance with leading CROs, pharma companies, and hospitals.
Hands-on training with real-world case studies.