PG-Diploma In Drug
Regulatory Affairs
12 Months Program
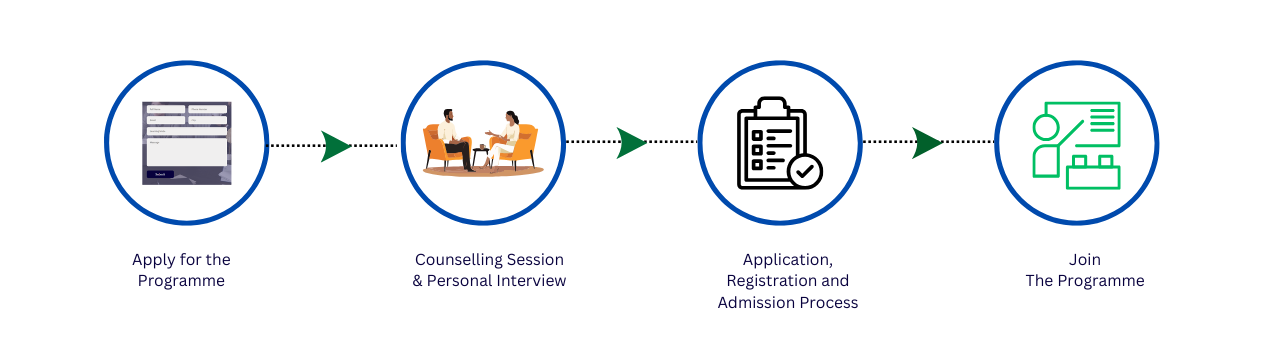
The Post Graduate Diploma in Drug Regulatory Affairs (PGDDRA) is a specialized one-year program designed to equip aspiring professionals with in-depth knowledge and practical skills in the regulatory domain. This program bridges the gap between academic learning and industry requirements, preparing students for a thriving career in pharmaceuticals, medical devices, biotechnology, and global regulatory bodies.
Learning Mode:
Both Residential and Distance learning modes are available, Each offering equal value, flexibility, and recognition.
Residential (Classroom)

Distance (Online)
Course Description:
The Post Graduate Diploma in Drug Regulatory Affairs (PGDDRA) offered by IILS is a comprehensive, one-year program thoughtfully structured into 4 semesters, each spanning 3 months. This program is designed to empower students with a strong academic and practical foundation in global regulatory affairs, keeping pace with evolving industry needs across pharmaceuticals, biotechnology, medical devices, and regulatory consultancy services.
Across 16 specialized modules, the curriculum covers the full scope of regulatory sciences—from the fundamentals of drug development and regulatory systems to the nuances of regulatory submissions, product registration, and compliance across international markets (USFDA, EMA, CDSCO, MHRA, TGA, WHO, etc.). The program begins with a foundational understanding of the regulatory landscape and progresses into global dossier requirements, clinical trial regulations, quality systems, GMP, GCP, regulatory intelligence, and lifecycle management.
Designed to blend theoretical instruction with practical exposure, the program incorporates real-world case studies, documentation exercises, and insights from regulatory experts to ensure students are industry-ready. By the end of the program, graduates gain not just academic knowledge but also critical regulatory thinking, technical proficiency, and confidence to navigate complex approval pathways in global markets.
At the end of each semester, candidates are required to appear for an internal assessment (semester examination). A minimum score of 50% is essential to successfully pass and advance to the next semester. This continuous evaluation approach ensures academic discipline and progressive learning throughout the course.
Course Curriculum:
SEMESTER I
- Module 1: Introduction to Regulatory Affairs
- Module 2: Drug Development and Approval Process
- Module 3: Regulatory Bodies & ICH Guidelines
- Module 4: Regulatory Submissions
SEMESTER II
- Module 5: Labelling, Packaging, and Advertising Regulations
- Module 6: Regulatory for Biologics, Medical Devices & OTCs
- Module 7: Post-Approval Changes and Lifecycle Management
- Module 8: Regulatory Intelligence and Strategy
SEMESTER III
- Module 9: Audits, Inspections, and Compliance
- Module 10: Emerging Markets and Trends
- Module 11: Dossier preparation in CTD format, eCTD submissions
- Module 12: Indian GMP Regulations
SEMESTER IV
- Module 13: AYUSH Regulatory Affairs
- Module 14: Regulatory Toxicology
- Module 15: Pharmaceutical Industry IPR
- Module 16: Industry Based Case Studies
Eligibility Requirements
Educational Qualifications
- Bachelor’s and Master’s degree in Life Sciences or related fields | B.Pharm | M.Pharm | Pharm-D | BDS | B.A.M.S|
- B.Sc Nursing | B.Sc Biotech | M.Sc Biotech | Microbiology | Zoology| Medicine | Biochemistry | M.B.B.S
Candidates who have passed with a minimum of 50% Graduate or Postgraduate + Internal Assessment + P. Interview

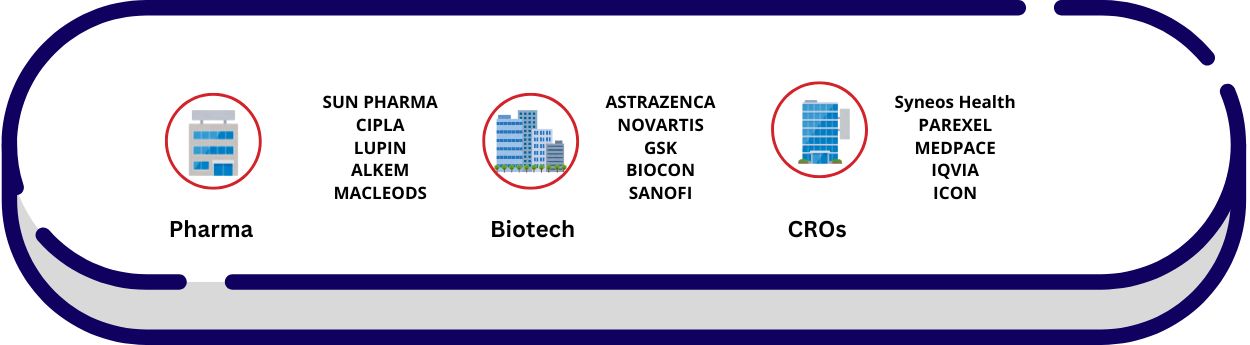
Industries Involved
Pharmaceutical:
- e.g., Drug development
Biotechnology
- e.g., Medical product innovation
Medical Device Companies
- e.g., Development and testing of devices like implants, diagnostic tools
CROs
- Contract Research Organizations for outsourcing research

Market Size & Industry Scope
The global Drug Regulatory Affairs market is projected to reach USD 22–25 billion by 2030, growing at a CAGR of 6–8%, driven by the expanding pharmaceutical, biotechnology, and medical device sectors. With increasing complexity in global compliance requirements, the demand for skilled regulatory professionals is at an all-time high.
- India, being a hub for drug manufacturing and clinical trials, is witnessing rapid regulatory evolution, opening vast career opportunities in regulatory consulting, dossier preparation, submissions, and compliance.
- Regulatory affairs professionals play a critical role in securing product approvals across international markets like USFDA, EMA, MHRA, CDSCO, TGA, and WHO PQ.
- Career prospects span across pharma companies, CROs, regulatory agencies, medical device firms, and global compliance consultancies.
High Demand, Low Supply: Despite being mission-critical, regulatory affairs remains a niche skill area with limited trained professionals, making it a strategic and rewarding career path.
Where DRA Professionals Work?
Career Prospects
Graduates of the PGDAR program can pursue roles such as:

- Regulatory Affairs Associate
- Regulatory Affairs Executive
- Regulatory Submission Specialist
- Regulatory Compliance Coordinator
- Regulatory Intelligence Analyst
- Dossier Submission Executive
- CMC Regulatory Executive
- Labeling & Compliance Officer
- Product Lifecycle Coordinator
- Regulatory Affairs Consultant
Salary Packages (India & Global)
| Level | Experience | Average Salary (INR/Year) | Global Package (USD/Year) |
|---|---|---|---|
| Entry Level | 0–2 Years | ₹3.5 – ₹5.5 LPA | $40,000 – $55,000 |
| Mid-Level | 3–6 Years | ₹6 – ₹10 LPA | $60,000 – $90,000 |
| Senior Level | 7+ Years | ₹12 LPA & above | $100,000+ |
Why Choose PGDAR at IILS?
Industry-focused curriculum aligned with regulatory standards.
Expert faculty with extensive Drug Regulatory experience.
Hands-on training with real-world case studies.
Placement assistance with leading Pharma, Biopharma companies.