PG-Diploma In Clinical Research (PGDCR)
12 Months Program
The Post Graduate Diploma in Clinical Research (PGDCR) is a specialized one-year program designed to equip aspiring professionals with in-depth knowledge and practical skills in the clinical research domain. This program bridges the gap between academic learning and industry requirements, preparing students for a thriving career in pharmaceuticals, biotechnology, contract research organizations (CROs), and healthcare institutions.
Learning Mode:
Both Residential and Distance learning modes are available, Each offering equal value, flexibility, and recognition.
Residential (Classroom)

Distance (Online)
Course Description:
The Post Graduate Diploma in Clinical Research (PGDCR) offered by IILS is a comprehensive, one-year program thoughtfully structured into 4 semesters, each spanning 3 months. It is designed to provide students with a robust academic and practical foundation in clinical research while aligning with current industry demands across pharmaceuticals, biotechnology, Medical Device Organization, CROs, and healthcare sectors.
Across 16 in-depth modules, the program covers a full spectrum of clinical research knowledge—from fundamentals to advanced applications. The journey begins with an introduction to clinical research, pharmacology, and ethical and regulations, gradually advancing into study design, regulatory requirements, project management, and trial execution. As the program progresses, students gain specialized exposure to data management, medical writing, biostatistics, and quality assurance—essential for real-world clinical trial environments.
The curriculum integrates theoretical understanding with practical insights, case-based discussions, and industry-relevant tools to ensure graduates are career-ready. By the end of the program, students are equipped not only with subject mastery but also with critical thinking, analytical, and operational skills needed to excel in the evolving landscape of clinical research.
At the end of each semester, candidates must appear for an internal assessment (semester examination). A minimum score of 50% is required to successfully pass and progress to the next semester. This evaluation process ensures academic accountability and a consistent pace of learning throughout the program.
Course Curriculum:
SEMESTER I
- Module 1: Introduction to Clinical Research
- Module 2: Pharmacology and New Drug Development
- Module 3: Clinical Research Ethics and Regulations
- Module 4: Study Design and Protocol Development
SEMESTER II
- Module 5: Clinical Trial Operations
- Module 6: Participant Recruitment and Informed Consent
- Module 7: Clinical Research Project Management
- Module 8: Data Collection and Management
SEMESTER III
- Module 9: Regulatory Affairs in Clinical Research
- Module 10: Advanced Topics in Clinical Research
- Module 11: Emerging Trends and Future Directions
- Module 12: Drug Safety Management
SEMESTER IV
- Module 13: Clinical Data Management
- Module 14: Medical Writing
- Module 15: Biostatistics and Data Analysis
- Module 16: Quality Assurance
Eligibility Requirements
Educational Qualifications
- Bachelor’s and Master’s degree in Life Sciences or related fields | B.Pharm | M.Pharm | BDS | B.A.M.S|
- B.Sc Nursing | B.Sc Biotech | M.Sc Biotech | Microbiology | Zoology| Medicine | Biochemistry | M.B.B.S
Candidates who have passed with a minimum of 50% Graduate or Postgraduate + Internal Assessment + P. Interview

Industries Involved
Pharmaceutical:
- e.g., Drug development
Biotechnology
- e.g., Medical product innovation
Medical Device Companies
- e.g., Development and testing of devices like implants, diagnostic tools
CROs
- Contract Research Organizations for outsourcing research

Growing Demand for Clinical Research Professionals
Market Size Overview:
- 2023 Value: USD 57.76 billion
- Projected Growth: From USD 61.58 billion in 2024 to USD 106.78 billion by 2032
- CAGR (2024-2032): 7.1%
Growth by Region:
- The Asia Pacific region is expected to grow at the highest CAGR during the forecast period.
Drivers of Market Growth
- Increased R&D investment by pharmaceutical and biotechnological companies globally.
- Focus on developing novel treatments for chronic diseases.
- Rising demand for outsourcing of R&D activities to CROs.
- Strategic Collaborations:
- Example: In July 2021, the Beijing Illness Challenge Foundation (ICF) in China formed a strategic relationship with Parexel to improve patient participation in clinical trials for rare diseases.
Impact of COVID-19 on the Market
Challenges During COVID-19:
- Lockdowns and resource shortages resulted in delays or halts in clinical studies.
Post-COVID Recovery:
- Increased focus on developing drugs, vaccines, and test kits for SARS-CoV-2.
- Strategic partnerships with CROs became more prevalent to accelerate R&D.
Notable Partnerships:
- In January 2021, ICON plc, BioNTech, and Pfizer collaborated to develop a COVID-19 vaccine and provided clinical trial services for its development.
Clinical Trial Market Trends
Pharmaceutical and Biotechnological Companies Increasing R&D Investments
- Companies in medical devices, pharmaceuticals, and biopharmaceuticals are allocating significant resources to the development of new technologies and medications.
- Focus is on improving R&D efficiencies and developing novel therapeutics.
- Increased investment in R&D by pharmaceutical and biotechnology companies is expected to drive market growth.
Clinical Trial Market Growth Factor
Rising Prevalence of Chronic Diseases
- Increased incidence of chronic diseases like diabetes, cancers, neurological disorders, and arthritis is driving demand for the development of effective therapeutics.
- Example: In June 2023, over half a billion people globally live with diabetes, projected to reach 1.30 billion in the next 30 years (Source: University of Washington).
- Cancer Prevalence: Europe accounted for 22.8% of all cancer cases in 2020 (Source: Globocan).
Rising Number of Clinical Trials Globally
- Annual clinical trial registrations have increased to meet the rising demand for treating chronic diseases.
- Example: WHO reported an 11.7% increase in registered clinical trials from 2020 to 2021.
Restraining Factors in Clinical Trials
Limited Availability of Skilled Workforce
- The industry faces a shortage of experienced professionals in clinical research.
- Competition among companies for qualified scientists is driving up costs, especially for smaller organizations.
High Costs of Clinical Trials
- The cost of drug development is substantial, averaging USD 2.60 billion per new drug (Source: Tufts Center for the Study of Drug Development).
- Only 12% of drug candidates in clinical trials gain U.S. FDA approval.
- Complexity in study design, lengthy time frames, and regulatory requirements also contribute to the high costs and time involved in clinical trials.
Clinical Trials in India
- Current Clinical Trials: 74,516 clinical trials registered and ongoing in India (Source: CTRI).
- Market Size (2022): Estimated at USD 2.07 billion.
- Projected Market Size (2030): Expected to reach USD 3.88 billion.
- Cost-effective trial strategies and a simplified regulatory system.
- Trained professionals in clinical research, medicine, and IT support.
- Availability of vulnerable populations, diverse patient groups, and experienced physicians.
- India has the potential to increase global clinical trials by 5x in the coming years.
- Current participation in global trials is just 3.2%, while the country contributes over 15% of the global burden for many prevalent diseases (e.g., respiratory infections, cardiovascular, diabetes, cervical cancer).
Workspaces of Clinical Research Personnel
Where Clinical Research Professionals Work?
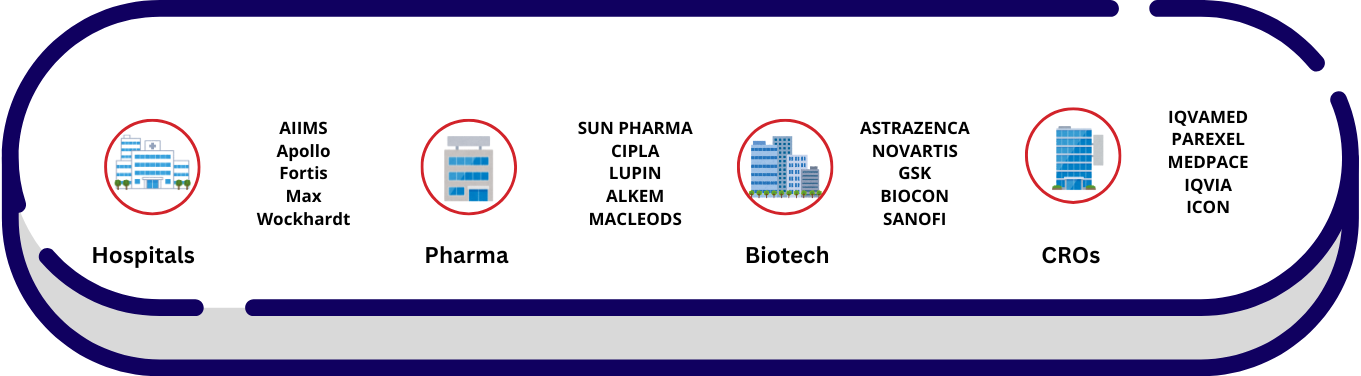
&...Central Labs_Global Central Labs


India’s Leading Central Labs

Types of Roles in CR Domain
Graduates of the PGDCR program can pursue roles such as:
At Hospital_the Investigative Site
The location(s) at or from where trial-related activities are conducted under the investigator’s/institution’s supervision.
- Principal Investigator (PI)
- Sub-Investigator (Sub-I)
- Clinical Research Coordinator (CRC)
- Clinical Research Nurses (CRN)
- Clinical Research Pharmacists (CRP)

Principal Investigator (PI)

A person responsible for the conduct of the clinical trial, including the trial participants for whom that person has responsibility during the conduct of the trial. If a trial is conducted by a team of individuals, the investigator is the responsible leader of the team and may be called the principal investigator. Where an investigator/institution is referenced in this guideline, it describes expectations that may be applicable to the investigator and/or the institution in some regions. Where required by the applicable regulatory requirements, the “investigator” should be read as “investigator and/or the institution.”
Responsibilities:
- Direct interaction with research subjects to ensure informed consent.
- Monitor safety and well-being of participants.
- Ensure adherence to study protocol and regulations.
- Address any patient concerns related to the trial.
Sub-Investigator (Sub-I)
Responsibilities:
- Assists the PI in conducting the clinical trial.
- Interacts with patients during visits to ensure protocol compliance.
- Provides clinical care and supervises patient safety.
- Acts as a secondary point of contact for subjects, supporting the PI.

Clinical Research Coordinator (CRC)

Average Salary Ranges (₹ 15,000 – ₹ 35,000)
Clinical Research Coordinator (CRC) is responsible for overseeing and managing the day-to-day operations of clinical trials. They support the Principal Investigator (PI) and Sub-Investigator (Sub-I) in all aspects of the study, including start-up, ethics approval, scheduling patient visits, assisting with data collection, managing study documents, and addressing issues identified during monitoring visits. CRCs play a key role in ensuring that clinical trials are conducted in compliance with the study protocol, regulatory requirements, ethical standards, and Good Clinical Practices (GCPs).
Responsibilities:
- Conduct patient recruitment and screening.
- Monitor patient health and adherence to trial protocols.
- Educates patients about study procedures, medications, and side effects.
- Ensures accurate documentation of patient interactions.
Clinical Research Nurse (CRN)
Responsibilities:
- Administers treatments and conducts medical assessments.
- Observes and reports patient reactions, side effects, and progress.
- Acts as a bridge between patients and the clinical research team.
- Ensures patients’ questions about the trial are answered.

Average Salary Ranges (₹ 20,000 – ₹ 41,000)
Clinical Research Pharmacists (CRP)

Clinical Research Pharmacists (CRPs) are responsible for providing specialized pharmacy expertise in the development, management, and handling of investigational products at both the program and protocol levels. Their primary objective is to ensure the safe and effective use of investigational products at clinical sites, including the creation of detailed pharmacy manuals for use by healthcare personnel.
Responsibilities:
- Dispenses and monitors investigational products.
- Educates patients on the proper use of trial medications.
- Monitors for potential drug interactions and adverse reactions.
- Ensures patient safety through proper medication administration.
Average Salary Ranges (₹ 15,000 – ₹ 45,000)
Ethics Committee (EC) Coordinator
An Ethics Committee (EC) Coordinator is responsible for managing the activities, operations, and administration of the ethics committee, which oversees the ethical standards and compliance of clinical trials and other research activities within institutions such as hospitals, universities, or corporations.
Responsibilities:
- AdminAgenda Preparation & Meeting Coordination
- Document Management
- Communication with EC Members
- Compliance & Guidelines
- Coordination with CRCs & Site Head
- Chennai Ethics Committee (CEC) Specific Duties
- Document Verification & Acknowledgment
Average Salary Ranges (₹ 25,000 – ₹ 75,000)

The Types of Designations That Exist In Operation

Sponsor | Operations
Within Industry (Biopharmaceutical / Medical Device Companies, Contract Research Organizations [CROs]
- Director of Clinical Operations
- Clinical Operations Manager
- Project Manager (Clinical Trials)
- Associate Project Manager (ACPM)
- Clinical Research Associate (CRA)
- Clinical Trial Assistant (CTA)
Director of Clinical Operations
The Director of Clinical Operations is a senior-level professional responsible for overseeing the planning, execution, and management of clinical trials within a pharmaceutical, biotech, or healthcare organization. They ensure that clinical trials are conducted efficiently, within budget, on schedule, and in compliance with regulatory and ethical guidelines, such as Good Clinical Practice (GCP). The Director of Clinical Operations plays a pivotal role in leading cross-functional teams, managing trial logistics, and aligning clinical strategies with the organization’s goals.
Responsibilities:
- Strategic Planning & Oversight
- Team Leadership & Management
- Budget Management
- Regulatory Compliance
- Vendor and CRO Management
- Risk Management & Problem-Solving
- Monitoring & Reporting
- Process Improvement
- Stakeholder Communication

Average Salary Ranges (₹8.0 Lakhs to ₹ 93.0 Lakhs )
Clinical Operations Manager

Average Salary Ranges (₹5.0 Lakhs to ₹ 40.0 Lakhs )
A Clinical Operations Manager is responsible for overseeing the day-to-day operations of clinical trials. They manage the clinical trial process, ensuring that trials are conducted in compliance with regulatory requirements, timelines, and budgets. This role includes coordinating teams, managing resources, and liaising between different stakeholders such as investigators, sponsors, and regulatory authorities. They ensure the smooth and efficient execution of clinical studies and play a critical role in ensuring the success of clinical trials within a research organization or pharmaceutical company.
Responsibilities:
- Team Management & Leadership
- Budget & Resource Management
- Regulatory Compliance
- Vendor Coordination
- Risk Assessment & Mitigation
- Monitoring & Reporting
- Process Improvement
- Stakeholder Communication
Project Manager (Clinical Trials)
A Project Manager (PM) is responsible for overseeing the planning, execution, and completion of clinical trials. They ensure that clinical studies are conducted on time, within budget, and according to regulatory requirements. The role involves coordinating cross-functional teams, managing resources, and maintaining communication between sponsors, clinical sites, and regulatory bodies. The Project Manager ensures the trial progresses smoothly, addressing challenges and risks to ensure successful study outcomes.
Responsibilities:
- Team Management & Leadership
- Budget & Resource Management
- Regulatory Compliance
- Vendor Coordination
- Risk Management & Problem-Solving
- Monitoring & Reporting
- Process Improvement
- Stakeholder Communication

Average Salary Ranges (₹5.0 Lakhs to ₹ 25.0 Lakhs )
Associate Project Manager (ACPM)

Average Salary Ranges (₹5.0 Lakhs to ₹ 25.0 Lakhs )
An Associate Project Manager (APCM) in clinical trials supports the Project Manager (PM) in overseeing the planning, execution, and completion of clinical studies. They assist in ensuring that clinical trials run smoothly, on time, and within budget. The Associate Project Manager is responsible for handling day-to-day project tasks, coordinating with cross-functional teams, and maintaining communication with stakeholders to ensure the success of the trial. This role is often an entry- to mid-level position, providing an opportunity for growth into full project management.
Responsibilities:
- Project Coordination & Support
- Team Collaboration
- Budget & Resource Tracking
- Regulatory Documentation & Compliance
- Vendor & CRO Support
- Monitoring & Reporting
- Stakeholder Communication
- Administrative Support
Clinical Research Associate (CRA)
A Clinical Research Associate (CRA) is a vital member of the clinical trials team, responsible for monitoring the progress of clinical trials at various sites. They ensure that the trials are conducted according to the study protocol, regulatory guidelines, and Good Clinical Practice (GCP). CRAs are responsible for overseeing data collection, patient safety, compliance with regulatory standards, and reporting of trial progress to sponsors. They play a crucial role in ensuring the quality and integrity of clinical trial data.
Responsibilities:
- Site Monitoring & Management
- Data Management & Reporting
- Regulatory Compliance & Documentation
- Site Support & Training
- Collaboration with Study Team
- Study Progress Tracking

Average Salary Ranges (₹ 35,000 – ₹ ₹ 2.5 Lakhs)
Clinical Trial Assistant (CTA)

Average Salary Ranges (₹ 25,000 – ₹ 50,000)
A Clinical Trial Assistant (CTA) supports the clinical trial team with administrative and operational tasks throughout the lifecycle of clinical trials. The CTA is responsible for maintaining essential trial documentation, organizing meetings, and ensuring that the necessary regulatory and clinical trial documents are up to date and properly filed. They play a key role in facilitating communication between the clinical team and stakeholders.
Responsibilities:
- Document Management
- Trial Documentation & Filing
- Study Set-up & Support
- Coordination of Meetings & Communications
- Tracking & Reporting of Study Milestones
- Regulatory Submissions Support
- Study Progress Monitoring
- Preparing Study Reports
- Audit & Inspection Preparation
The Types of Designations In Clinical Data Manager
Sponsor | Operations
- Director of Data Management
- Clinical Data Manager (CDM)
- Senior Data Manager
- Lead Data Manager
- Data Management Associate
- Clinical Data Coordinator
- Clinical Data Analyst
- Data Entry Associate
- Data Quality Manager
- Database Programmer
- Clinical Database Administrator
- Clinical Data Reviewer
- Clinical Data Validator
- Data Standards Specialist
- Biostatistician

Clinical Data Manager (CDM)

A Clinical Data Manager (CDM) oversees the collection, processing, and analysis of data from clinical trials, playing a vital role in the development of new medical treatments by ensuring the accuracy, completeness, and regulatory compliance of trial data.
Clinical Data Managers collaborate with other members of the clinical research team, including Clinical Research Associates, Data Analysts, and Biostatisticians, to ensure that data management processes are executed efficiently and precisely. Their responsibilities include designing and implementing data management plans, developing guidelines for data entry, and overseeing quality control processes for the data collected.
Responsibilities:
- Data Collection & Management
- Design case report forms (CRFs)
- Database Design & Maintenance
- Data Cleaning & Validation
- Ensuring Data Accuracy & Integrity
- Regulatory Compliance & Standards
- Data Review & Query Resolution
- Collaboration with Cross-Functional Teams
- Audit & Inspection Support
- Data Export & Submission
Average Salary Ranges (₹ 45,000 – ₹ 2.5 lakh)
Clinical Data Coordinator (CDC)
A Clinical Data Coordinator (CDC) is responsible for supporting the management and processing of data collected during clinical trials. The CDC works closely with the Clinical Data Manager (CDM) to ensure that the data is collected, entered, and validated accurately, adhering to regulatory and quality standards.
Responsibilities:
- Data Entry & Management
- Data Validation & Cleaning
- Query Management & Resolution
- Collaboration with Clinical Teams
- Compliance with Regulatory Standards
- Supporting Data Audits & Inspections
- Support in Data Analysis

Average Salary Ranges (₹ 35,000 – ₹ ₹ 1.5 Lakhs )
Clinical Data Analyst (CDA)
A Clinical Data Analyst is responsible for analyzing and interpreting data collected from clinical trials. They ensure that the data is accurate, complete, and compliant with regulatory standards. Their work is crucial in identifying trends, validating trial outcomes, and supporting decision-making in clinical research.
Responsibilities:
- Data Analysis & Interpretation
- Data Cleaning & Validation
- Generating Reports & Insights
- Trend Analysis & Outcome Assessment
- Collaborating with Cross-Functional Teams
- Ensuring Data Integrity & Accuracy
- Compliance with Regulatory Standards
- Data Query & Resolution
- Supporting Statistical Analysis
- Contributing to Study Reports & Publications

Average Salary Ranges (₹ 35,000 – ₹ ₹ 1.5 Lakhs )
Quality Assurance

Sponsor | Operations
- Director of Quality Assurance
- Quality Assurance Manager
- Senior QA Specialist
- QA Associate
- QA Auditor
- Compliance Specialist
- GCP (Good Clinical Practice) Auditor
- Regulatory QA Specialist
- QA Coordinator
- Quality Control (QC) Manager
- QA Inspector
- Document Control Specialist
- QA Compliance Officer
- Quality Systems Manager
- Validation Specialist
Electronic Trial Master File (eTMF)
CRO| Operations-eTMF
- e-TMF Manager
- e-TMF Specialist
- TMF Coordinator
- TMF Administrator
- TMF Document Specialist
- TMF Associate
- TMF Compliance Lead
- e-TMF System Administrator
- TMF Quality Control Specialist
- e-TMF Project Manager
- TMF Archivist
- TMF Auditor
- Senior e-TMF Coordinator
- e-TMF Trainer
Medical Writing

Sponsor | Operations
- Director of Medical Writing
- Medical Writing Manager
- Senior Medical Writer
- Medical Writer
- Clinical Document Writer
- Regulatory Writer
- Medical Writing Associate
- Medical Writing Specialist
- Scientific Writer
- Statistical Writer
- Submission Writer
- Medical Copy Editor
- Clinical Research Writer
- Manuscript Writer
- Protocol Writer
- Clinical Report Writer
Drug Safety
Sponsor |CRO| Safety
- Director of Pharmacovigilance/Safety
- Drug Safety Manager
- Pharmacovigilance (PV) Officer
- Drug Safety Associate
- Senior Drug Safety Associate
- Pharmacovigilance Specialist
- Drug Safety Physician
- Safety Data Analyst
- Pharmacovigilance Scientist
- Medical Safety Officer
- Signal Detection Specialist
- Case Processing Specialist
- Adverse Event Reporting Coordinator
- Risk Management Specialist
- Safety Compliance Officer
- Safety Surveillance Manager
- Aggregate Reporting Specialist

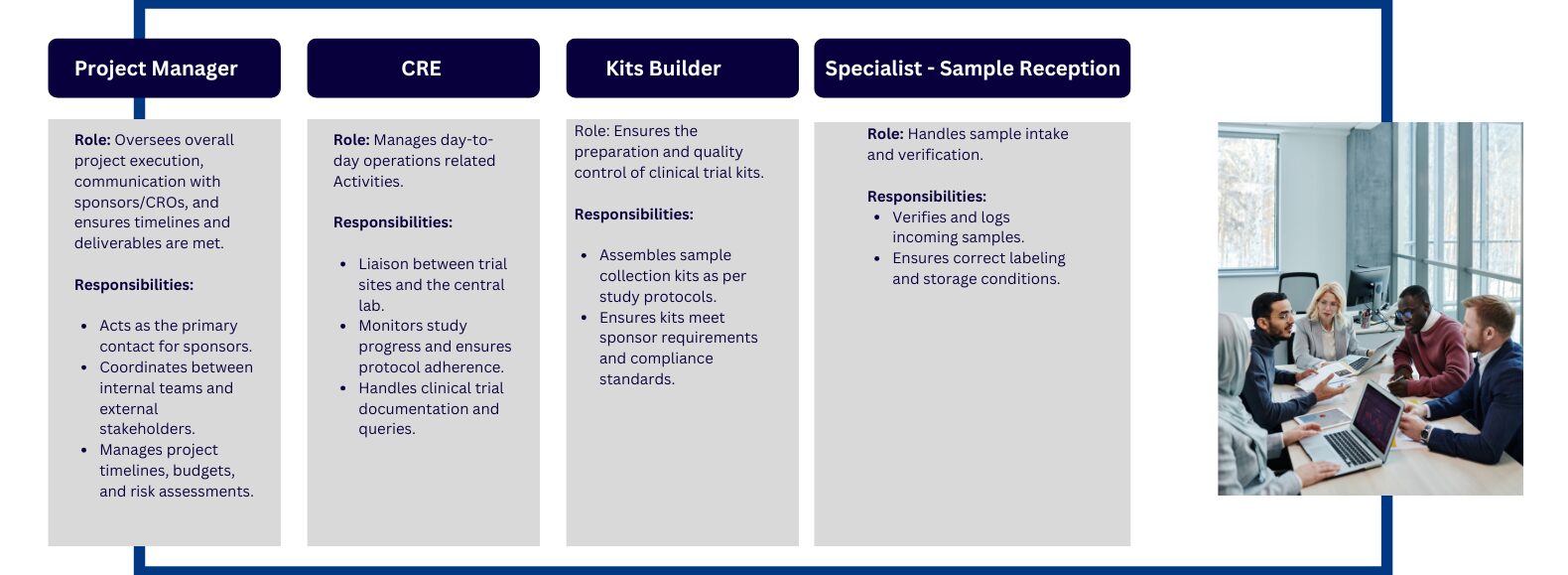
Designations That Exist in Central Lab
Why Choose PGDCR at IILS?
Industry-focused curriculum aligned with regulatory standards.
Expert faculty with extensive clinical research experience.
Placement assistance with leading CROs, pharma companies, and hospitals.
Hands-on training with real-world case studies.