PG-Diploma In Clinical SAS (PGDC-SAS)
12 Months Program
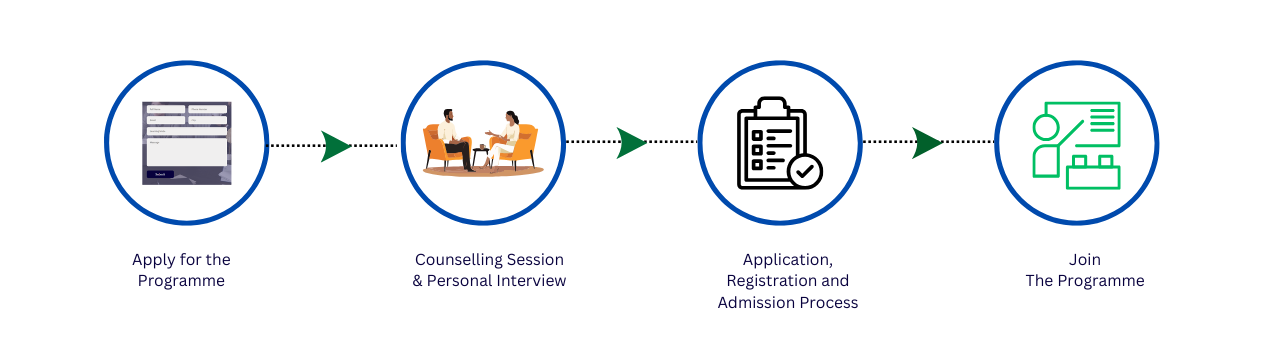
The Post Graduate Diploma in Clinical Statistical Analysis System “SAS” (PGDC-SAS) is a specialized 12 months program crafted to empower future professionals with comprehensive knowledge and hands-on training in SaaS applications used across the clinical research industry. This program bridges academic learning with real-world technological demands, preparing students for impactful roles in CROs, pharma, biotech, Hospital, and digital health organizations.
Learning Mode:
Both Residential and Distance learning modes are available, Each offering equal value, flexibility, and recognition.
Residential (Classroom)

Distance (Online)
Course Description:
The Professional Diploma in Clinical SaS (PDCS) offered by IILS is a comprehensive, 12-months program designed to develop expertise in SaaS-based technologies used in clinical research. The program is structured across 4 semesters of 3 months each, integrating academic foundations with hands-on tool-based learning tailored for careers in CROs, pharma, and health-tech industries.
Through 16 core modules, the curriculum covers the clinical development process, data lifecycle management, and statistical reporting using industry-standard SaaS platforms like Medidata Rave, Oracle Clinical, REDCap, Argus Safety, and Veeva Vault. Students gain knowledge in data standards (CDISC, SDTM, ADaM), regulatory frameworks (ICH-GCP, FDA, EMA), and generate submission-ready outputs such as Tables, Listings, and Figures (TFLs).
Designed to bridge theory and real-world application, the program incorporates tool demonstrations, project-based assignments, and mentorship by domain experts to ensure students are industry-ready. Graduates emerge with strong technical capabilities, analytical skills, and a practical understanding of digital clinical operations.
At the end of each semester, candidates are required to appear for an internal assessment (semester examination). A minimum score of 50% is essential to successfully pass and advance to the next semester and qualify for certification. This continuous evaluation approach ensures academic discipline and progressive learning throughout the course.
Course Curriculum:
SEMESTER I
- Module 1: Introduction to Clinical SAS
- Module 2: Introduction to Base SAS Programming
- Module 3: Introduction to Clinical Trials and CDM
- Module 4: Introduction to Reading & Combining SAS Data Sets
SEMESTER II
- Module 5: Introduction to Advanced SAS
- Module 6: Introduction to Real Time Macros
- Module 7: Introduction to Macro Variables
- Module 8: DATA Step and SQL Interfaces
SEMESTER III
- Module 9: Introduction to Macro Programs
- Module 10: Introduction to Special Purpose Domains-(SDTM)
- Module 11: Introduction to Interventions General Observation Class
- Module 12: Introduction to Events General Observation Class
SEMESTER IV
- Module 13: Introduction to Finding General Observation Class
- Module 14: Data Visualization and Graphs
- Module 15: Real Time Projects, Psychiatry | Dermatology
- Module 16: Real Time Projects, Oncology | Endocrionology
Eligibility Requirements
Educational Qualifications
- Bachelor’s and Master’s degree in Life Sciences or related fields | B.Pharm | M.Pharm | Pharm-D | BDS | B.A.M.S|
- B.Sc Nursing | B.Sc Biotech | M.Sc Biotech | Microbiology | Zoology| Medicine | Biochemistry | M.B.B.S
Candidates who have passed with a minimum of 50% Graduate or Postgraduate + Internal Assessment + P. Interview

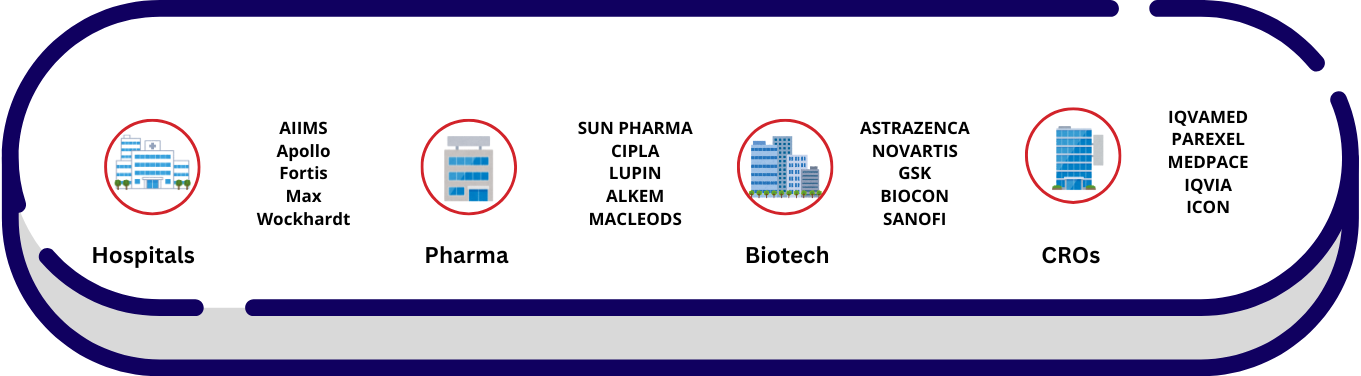
Industries Involved
Pharmaceutical:
- e.g., Drug development
Biotechnology
- e.g., Medical product innovation
Medical Device Companies
- e.g., Development and testing of devices like implants, diagnostic tools
CROs
- Contract Research Organizations for outsourcing research

Market Size & Industry Scope
The global clinical research and SaaS-enabled healthcare technology market is experiencing exponential growth. As of 2024, the Clinical Trial Management System (CTMS) and Clinical Data Management Software markets alone are valued at over USD 6.5 billion, projected to exceed USD 12 billion by 2030, with a CAGR of 12–15%. The rise in decentralized trials, digital health platforms, and AI-driven analytics has fueled demand for SaaS professionals skilled in regulatory-compliant, cloud-based clinical tools.
Pharmaceutical companies, Contract Research Organizations (CROs), biotech firms, and health-tech startups are rapidly adopting SaaS platforms for clinical data capture, trial monitoring, pharmacovigilance, and regulatory submissions. This digital transformation is creating a surge in opportunities for trained professionals who understand both clinical workflows and SaaS applications.
With India emerging as a major hub for global clinical trials and data operations, certified professionals in Clinical SaaS are uniquely positioned to work in roles such as: Clinical Data Analyst | SaaS Implementation Specialist | Statistical Programmer |CDISC/SDTM Specialist |eTMF & CTMS Coordinator |Clinical SaaS Trainer/Support Consultant
Graduates of the PDCS program will be equipped with domain-relevant, future-ready skills to thrive in this high-growth, tech-enabled segment of the life sciences industry.
Where SAS Professionals Work?
Career Prospects
Certified of the PGDC-SAS program can pursue roles such as:

- Clinical SAS programmer
- Business Analyst
- Data Analyst
- Statstical Analyst
- Data Scientist
- Reporting Analyst
- Healthcare Analyst
- Risk Analyst
- Marketing Analyst
- Drug Safety Analytics
- Clinical SAS + SQL Programmer
- Principal Statistical Programmer
- Principal SAS Programmer
- Data Analyst – Clinical Trials
- Senior Clinical SAS Programmer
- Clinical Data Manager (with SAS skills
- Director – Clinical Data Analytics
- VP – Data Sciences / Programming
- Clinical SAS Consultant
- Risk-Based Monitoring Analyst
India’s top companies actively hiring Clinical SAS professionals
🧪 India-Based CROs & Pharma
- Pharma
- Emcure Pharmaceuticals
- Cliantha Research
- Novotech
- QED Pharmaceutical Services
- Navitas Life Sciences
- Medpace, Inc.
- Fortrea
- MOLECULAR CONNECTIONS
- Element Technologies Inc.
- Clinotica
- Covalent Trainings
- Medidata Solutions
🌍 Global CROs & Pharma
- IQVIA
- Parexel
- ICON plc
- Cytel
- Sanofi
- Novartis
- Piramal
- Dr. Reddy’s
- Syneos Health
- Aurobindo Pharma
🏢 IT & Consulting Giants with Life‑Sciences Focus
- Cognizant
- Cognizant
- Accenture
- Wipro
- HCL
- Genpact
🏥 Health-Tech & Specialized Firms
- CitiusTech
- Indegene
Salary Packages (India & Global)
| Level | Experience | Average Salary (INR/Year) | Global Package (USD/Year) |
|---|---|---|---|
| Entry Level | 0–2 Years | ₹3.5 – ₹5.5 LPA | $40,000 – $55,000 |
| Mid-Level | 3–6 Years | ₹6 – ₹10 LPA | $60,000 – $90,000 |
| Senior Level | 7+ Years | ₹12 LPA & above | $100,000+ |
Note: Salaries may vary based on location, company size, certifications, and international experience.
Why Choose PGDC-SAS at IILS?
Industry-focused curriculum aligned with regulatory standards.
Expert faculty with extensive SAS experience.
Hands-on training with real-world case Insights.
100 % Placement assistance with leading Pharma, Biopharma companies.